1. 5 Tips To Design The Ultimate Mody 2 Trial Today

Introduction

Designing the ultimate Mody 2 trial can be an exciting and challenging task, as it requires careful planning and consideration of various factors. Whether you are a researcher, a medical professional, or a trial coordinator, creating an effective trial design is crucial for obtaining reliable and meaningful results. In this blog post, we will explore five essential tips to help you design a successful Mody 2 trial, ensuring its efficiency and impact.
Understanding Mody 2

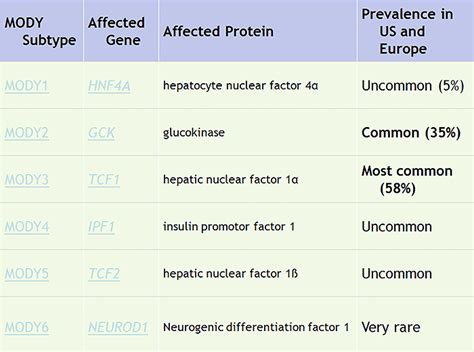
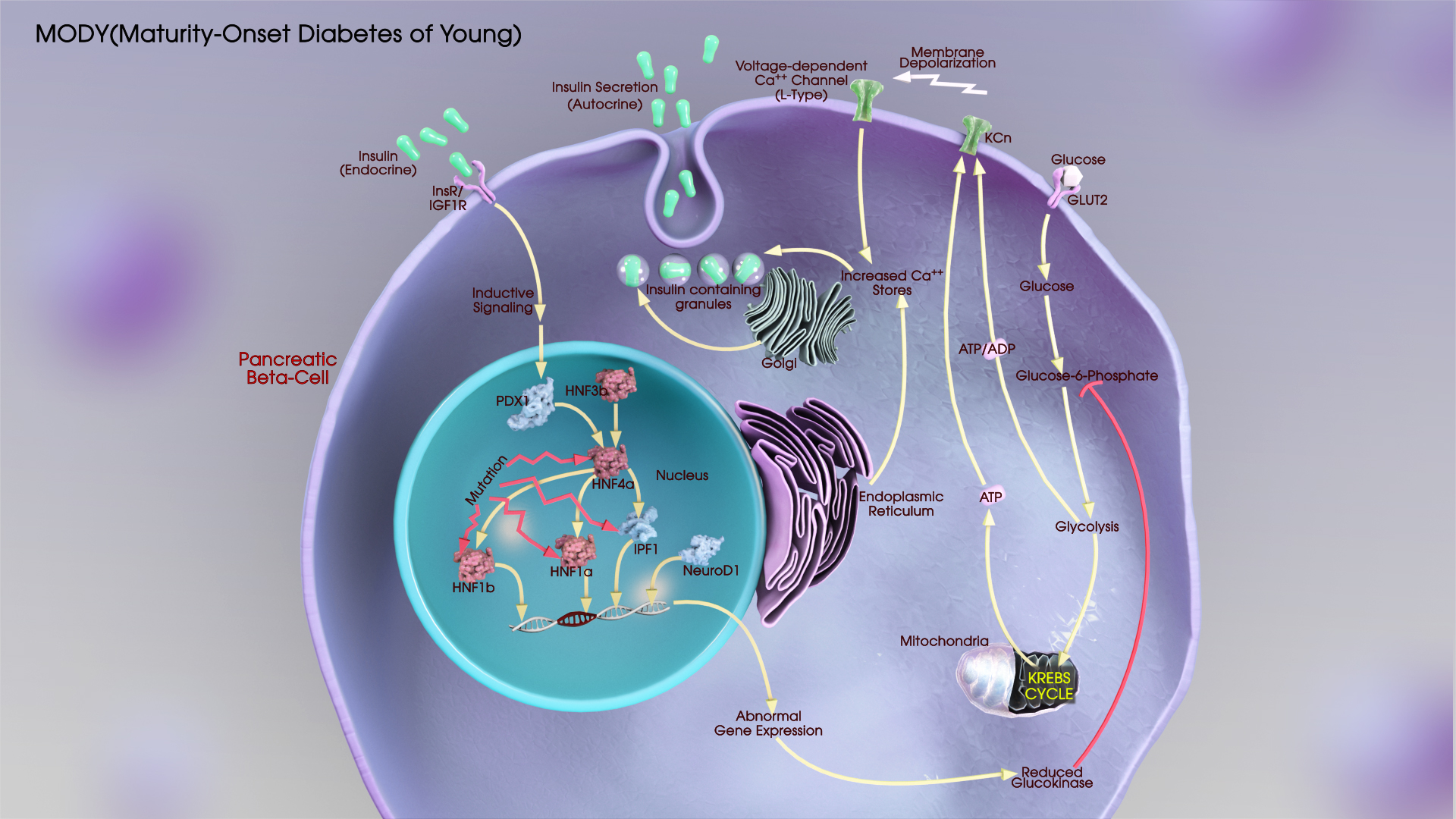
Before delving into the trial design, let’s briefly understand what Mody 2 is. Mody 2, or Maturity Onset Diabetes of the Young type 2, is a rare form of diabetes that typically develops during adolescence or young adulthood. It is caused by specific genetic mutations that affect insulin production and secretion. Designing a trial focused on Mody 2 aims to improve our understanding of this unique condition and find effective treatment strategies.
Tip 1: Define Clear Objectives

The first step in designing any trial is to define clear and specific objectives. For a Mody 2 trial, your objectives should revolve around answering key research questions related to the disease. Consider the following aspects:
- Research Question: Clearly state the primary research question you aim to address. For example, “What are the long-term effects of a specific treatment on Mody 2 patients?”
- Study Population: Define the target population for your trial. Specify the age range, gender, and any other relevant characteristics of the participants.
- Outcomes: Identify the primary and secondary outcomes you want to measure. These could include glycemic control, quality of life, or specific metabolic markers.
- Duration: Determine the appropriate duration for your trial based on the nature of Mody 2 and the research question.
Tip 2: Participant Recruitment and Eligibility

Recruiting the right participants is crucial for the success of your Mody 2 trial. Here are some considerations for participant recruitment and eligibility:
- Collaboration: Partner with healthcare facilities, diabetes clinics, and support groups specializing in Mody 2. Their expertise and patient networks can greatly facilitate recruitment.
- Inclusion Criteria: Establish clear inclusion criteria to ensure a homogenous study population. This may include age, genetic testing results, and specific clinical characteristics.
- Exclusion Criteria: Define exclusion criteria to avoid including participants who may introduce bias or complicate data analysis. For example, individuals with other forms of diabetes or certain comorbidities.
- Informed Consent: Ensure all participants provide informed consent, understanding the purpose, risks, and benefits of the trial.
Tip 3: Study Design and Randomization
The study design is a critical aspect of trial planning. For a Mody 2 trial, consider the following:
- Randomized Controlled Trial (RCT): RCTs are often considered the gold standard for clinical trials. Randomly assign participants to different treatment groups to minimize bias and ensure comparability.
- Blinding: Consider blinding participants, researchers, and/or outcome assessors to reduce the risk of bias. Double-blinding is ideal but may not always be feasible.
- Crossover Design: If appropriate, a crossover design can be used, where participants receive both treatments sequentially with a washout period in between.
- Sample Size: Calculate the required sample size based on statistical power calculations to ensure the trial is adequately powered.
Tip 4: Intervention and Treatment Protocols

The intervention or treatment protocol is the heart of your Mody 2 trial. Here are some key considerations:
- Treatment Options: Explore various treatment options, such as insulin therapy, oral medications, lifestyle interventions, or a combination of these.
- Dosing and Titration: Establish clear guidelines for dosing and titration of medications, considering individual patient needs and response.
- Adherence Monitoring: Implement strategies to monitor and promote treatment adherence, as poor adherence can affect trial outcomes.
- Safety Monitoring: Put in place robust safety monitoring protocols to identify and manage any adverse events promptly.
Tip 5: Data Collection and Analysis
Effective data collection and analysis are essential for drawing meaningful conclusions from your Mody 2 trial. Consider the following:
- Outcome Measures: Define the outcome measures you will use to assess the effectiveness of the intervention. Ensure they are relevant, valid, and reliable.
- Data Collection Tools: Choose appropriate data collection tools, such as questionnaires, electronic health records, or laboratory tests, to capture the necessary information.
- Data Management: Establish a robust data management system to ensure data integrity, confidentiality, and accuracy.
- Statistical Analysis: Develop a statistical analysis plan in advance, specifying the appropriate statistical tests and methods for data analysis.
Conclusion

Designing the ultimate Mody 2 trial requires careful planning, attention to detail, and a comprehensive understanding of the disease. By following these five tips—defining clear objectives, recruiting the right participants, implementing a robust study design, developing effective intervention protocols, and ensuring rigorous data collection and analysis—you can create a trial that generates valuable insights into Mody 2 management and treatment. Remember, a well-designed trial sets the foundation for advancing our understanding of this rare diabetes type and improving patient care.
📈 Note: Regularly review and update your trial design based on emerging evidence and feedback from participants and researchers.
FAQ
How long should a Mody 2 trial typically last?
+The duration of a Mody 2 trial can vary depending on the research question and the nature of the intervention. Typically, trials range from several months to a few years. It is essential to consider the long-term effects of the intervention and the progression of Mody 2 when determining the trial duration.
What are some common challenges in Mody 2 trials?
+Mody 2 trials may face challenges such as the rarity of the disease, which can make participant recruitment difficult. Additionally, the genetic basis of Mody 2 can present unique challenges in terms of identifying suitable interventions and interpreting results. Close collaboration with geneticists and diabetes specialists is crucial.
How can I ensure participant retention in a Mody 2 trial?
+Participant retention is vital for the success of any trial. To enhance retention, provide regular updates and support to participants, address any concerns promptly, and offer incentives or recognition for their contribution. Building a strong relationship with participants can improve trial completion rates.
What are the key ethical considerations in Mody 2 trials?
+Ethical considerations are paramount in Mody 2 trials, especially given the vulnerability of the study population. Ensure that informed consent is obtained, and participants fully understand the risks and benefits. Maintain confidentiality and protect participant data. Regularly review and monitor the trial for any ethical concerns.
How can I disseminate the findings of my Mody 2 trial effectively?
+Disseminating trial findings is crucial for advancing knowledge and improving patient care. Consider presenting your results at relevant conferences, publishing in peer-reviewed journals, and sharing insights with patient support groups and healthcare professionals. Effective dissemination ensures that your research has a broader impact.



